35+ Enthalpy Of Reaction Calculator
The Heat of Reaction also known and Enthalpy of Reaction is the change in the enthalpy of a chemical reaction that occurs at a constant pressure. First you multiply each molecules enthalpy of formation.

Temperature Regulated Cooling Dominates Warming And Why The Earth Stopped Cooling At 15 C Watts Up With That
Calculate the enthalpy of reaction ΔH To classify the net energy output or input of chemical reactions you can calculate something called the enthalpy change ΔH or heat of reaction.
. 0228 mol BaO limiting reagent 01853 mol Al Molar Ratio 123 -16698 - 3-5581 33441. The heat of reaction can be calculated based on the standard heat of formation of all reactants involved. ΔH ΔQ p ΔV.
The following formula can be used. A change in enthalpy. However it is usually determined by measuring the heat production over time using a.
P ΔH ΔQ ΔV. If you know these quantities use the following formula to work out. The most basic way to calculate enthalpy change uses the enthalpy of the products and the reactants.
The standard enthalpy of formation of substances is here. Determine the bond enthalpy of the products Hproducts H p r o d u c t s by using a bond enthalpy table. If I want to calculate the amount of energy heat released in.
Calculate the enthalpy change of a reaction with the Free Enthalpy Calculator. Determine the bond enthalpy of the reactants Hreactants H r e. Calculate enthalpy change when 350 grams barium oxide reacts with 500 grams of aluminum.
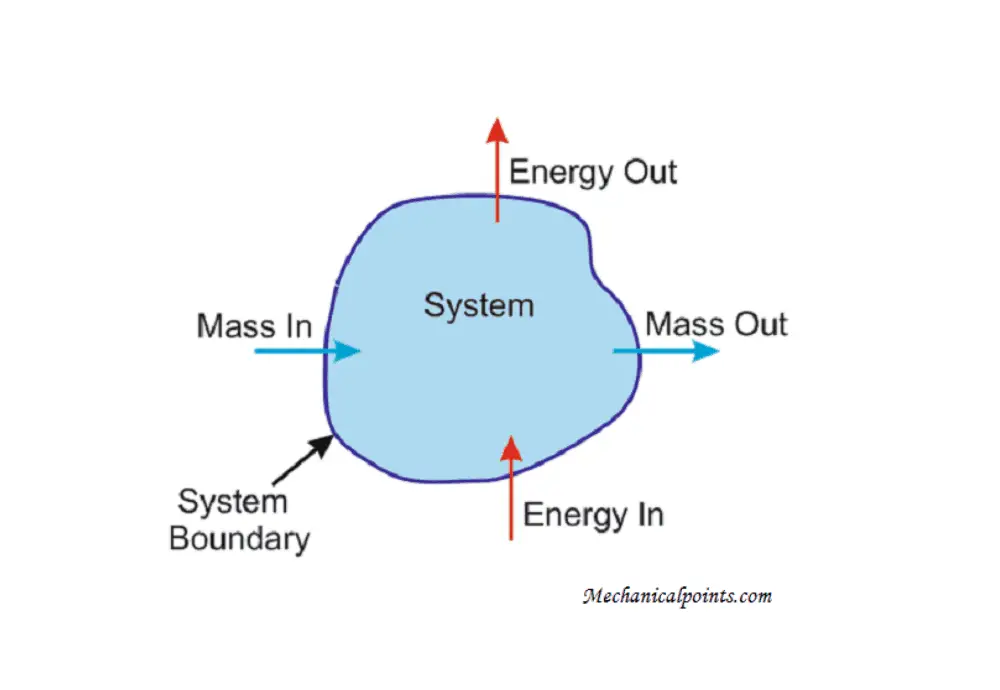
The precise definition of enthalpy H is the sum of the internal energy U plus the product of pressure P and volume V. In symbols this is. Yes per mole of a reaction as written to my thinking that is the right interpretation of the unit kJ mol.
By making considerable change in enthalpy equation we get. To use this online calculator for Heat Transfer in Thermochemical Reaction enter Mass M flight path Specific Heat Capacity c Change in Temperature T and hit the calculate button. However an online Chemical Equation Balancer Calculator.
The reaction scheme and the enthalpy formula are two easy ways of calculating the enthalpy change. The heat of combustion of benzene in a bomb calorimeter ie constant volume at 25C was found to be 32639 kJ mol-1. Change in enthalpy formula is ΔH reaction ΔH f products - ΔH f reactants Therefore change in enthalpy of the.
It is a thermodynamic. Calculate the heat of combustion of. Enthalpy is defined as the heat absorbed by a system and the total work done while expanding.
H rxn H fproducts H freactants. The Enthalpy of chemical reaction at absolute temperatures is defined as the difference in activation energy between products and reactants for forward and backward reactions at. H U PV.
P 7 41 11. To do this you need to use an enthalpy formula known as Hesss Law.
Chemical Thermodynamics
How To Spell I Love You On A Calculator Quora

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow
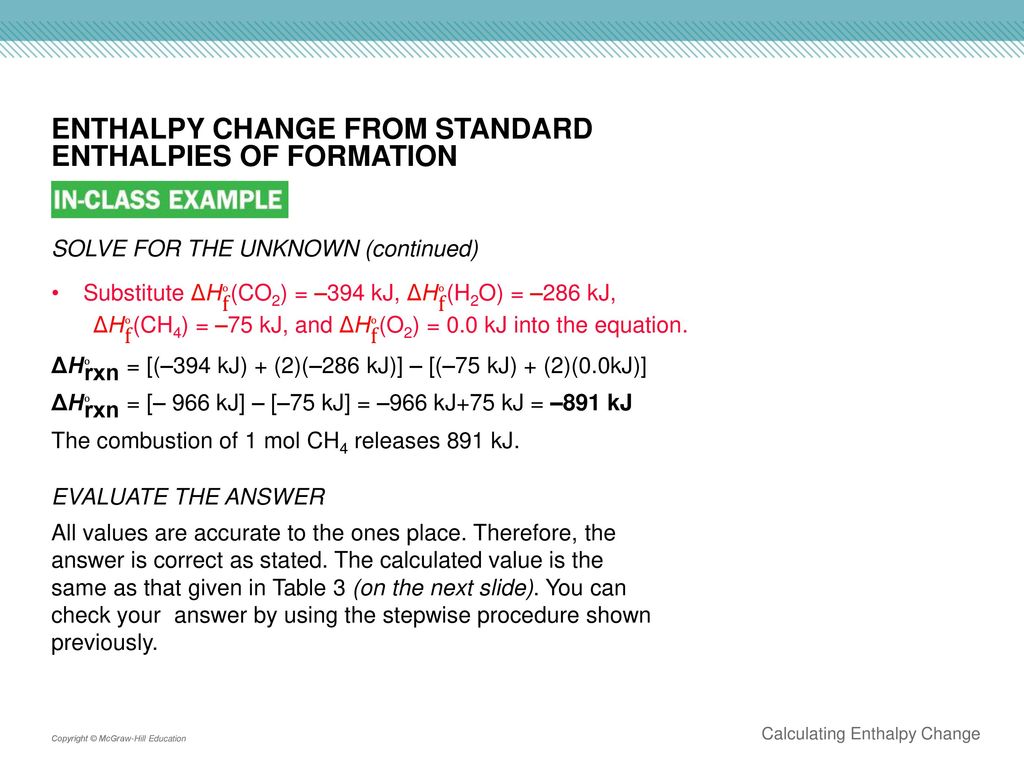
Section 4 Calculating Enthalpy Change Ppt Download

Enthalpies Of Formation Chemsitry Tutorial Youtube
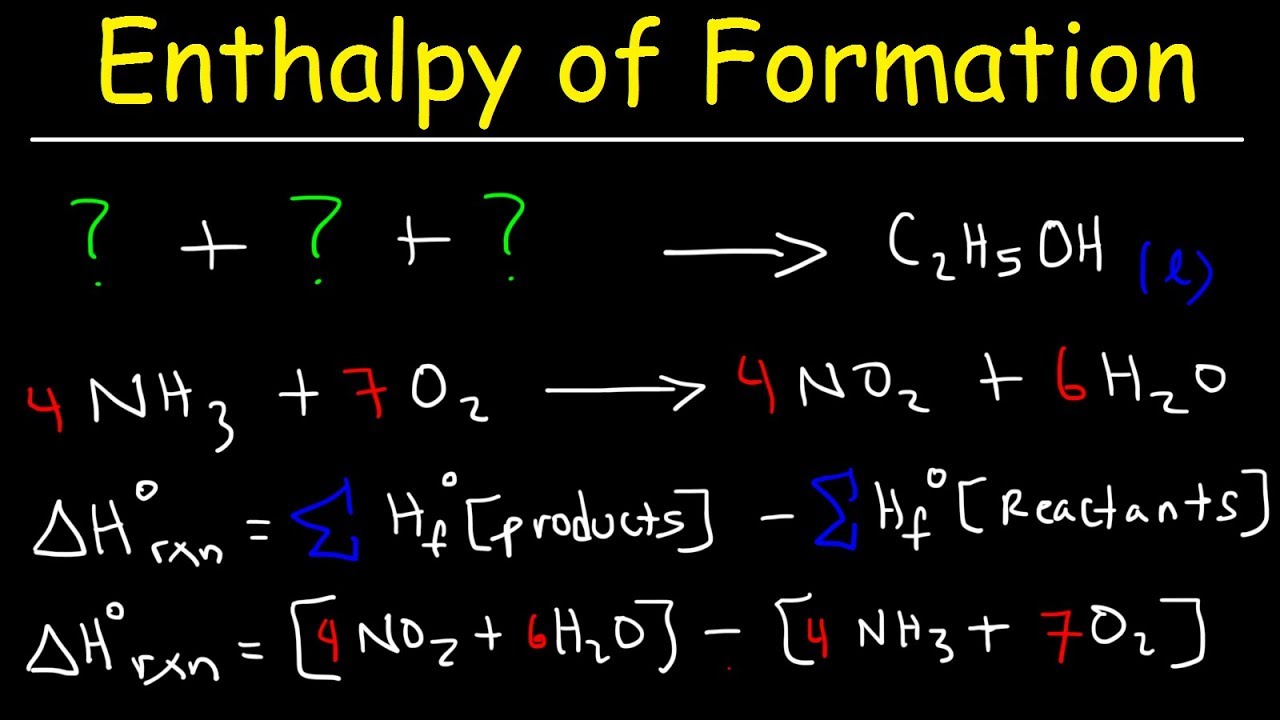
Enthalpy Of Formation Reaction Heat Of Combustion Enthalpy Change Problems Chemistry Youtube
Chemical Thermodynamics

Topic 5 Energetics Msjchem Tutorial Videos For Ib Chemistry
Chemical Thermodynamics
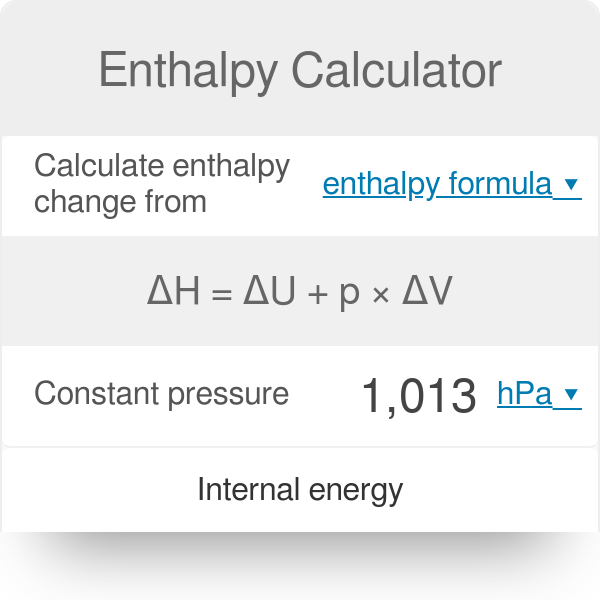
Enthalpy Calculator 100 Free Calculators Io
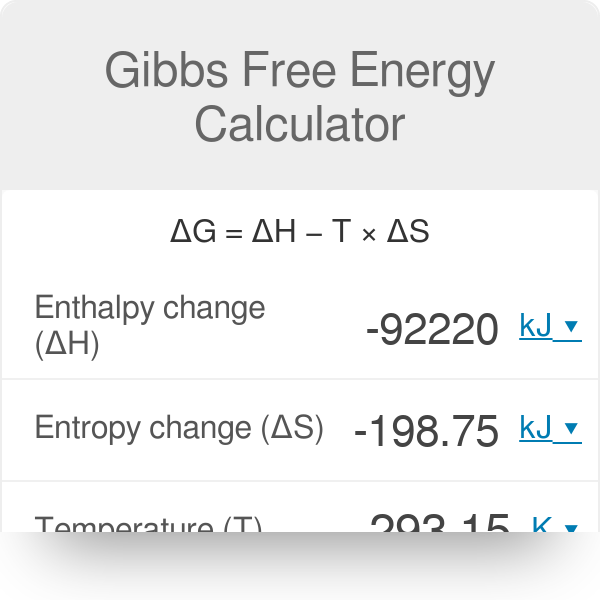
Gibbs Free Energy Calculator

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow

Isokinetic Relationship Isoequilibrium Relationship And Enthalpy Entropy Compensation Chemical Reviews
Chemical Thermodynamics
Physical Chemistry Calculations Involving Enthalpy